Immatics Announces European Clinical Expansion of its Adoptive Cell Therapy Programs
- First patient has been treated in Germany in Immatics’ ACTengine® IMA202-101 trial
- German regulatory agency, Paul-Ehrlich-Institute (PEI), granted approval to commence another clinical ACTengine® trial in Germany investigating Immatics’ IMA203 product candidate
- Three clinical trial sites in Germany have started recruiting patients for Immatics’ ACTengine® IMA200 trial series
Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell redirecting cancer immunotherapies, announced today the treatment of the first patient in the IMA202-101 trial in Europe following the Clinical Trial Application (CTA, the equivalent of an IND approval by FDA) approval by Paul-Ehrlich-Institute (PEI), the regulatory body for cell and gene therapies in Germany. In addition, Immatics has been granted regulatory approval by PEI to initiate another phase I clinical trial in Germany to evaluate safety, tolerability and initial signs of clinical efficacy of IMA203.
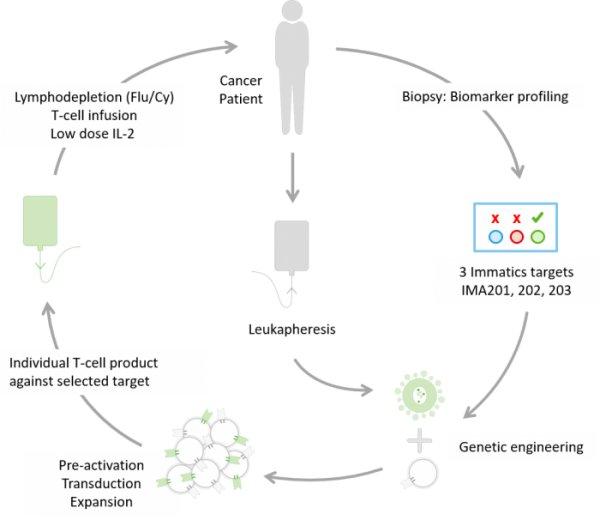
The clinical trials of the IMA200 series will investigate up to three novel cancer immunotherapies, which include IMA202 (NCT03441100) and IMA203 (NCT03686124). IMA202 and IMA203 are designed to target unique peptides derived from either melanoma-associated antigen 1 (“MAGEA1”) or preferentially expressed antigen in melanoma (“PRAME”), respectively. Both are built on Immatics’ proprietary ACTengine® approach in which the patient’s own T cells are genetically engineered to express an exogenous T cell receptor (TCR) directed against true cancer targets. By introducing this novel cancer specific TCR, the goal is to redirect and selectively activate the patient’s T cells to fight the tumor. The studies will investigate the safety and tolerability of Immatics’ Adoptive Cell Therapies (ACT) in patients with target-positive solid cancers and seek initial signals of anti-tumor activity. Moreover, persistence of the infused engineered T cells will be monitored in the patient’s blood as T cell persistence is considered a major pre-requisite to obtain an anti-tumor response. The aim is to develop innovative personalized immunotherapies targeting a patient’s tumor selectively and effectively.
The initial group of clinical trial sites in Germany includes the University Hospital Carl Gustav Carus in Dresden, the University Hospital Bonn and the University Hospital of Würzburg. Previous patients in the IMA200 series were initially enrolled at The University of Texas MD Anderson Cancer Center in Houston, Texas, and more recently at the Columbia University Irving Medical Center in New York and the UPMC Hillman Cancer Center in Pittsburgh, Pennsylvania.
Cedrik Britten, MD, Chief Medical Officer of Immatics commented: “As part of our strategy to increase the geographical foot-print for our clinical sites, we are currently expanding them in the US and in Europe. We are delighted to have gained a new regulatory approval from PEI and to have treated the first patient in Germany. This expansion elevates our clinical organization to a global level and adds operational flexibility that has become even more important in light of the global COVID-19 pandemic. We look forward to continuing to collaborate with leading clinicians to advance our mission of delivering the power of T cells to cancer patients on both sides of the Atlantic.”
Dr. Martin Wermke, Coordinating Investigator and Head of the Early Clinical Trial Unit of the National Center for Tumor Diseases Dresden (NCT/UCC) at the University Hospital Carl Gustav Carus in Dresden, Germany, commented: “Having been involved since the early stages of this clinical research, I am excited to witness the next phase of development of this fascinating pipeline of immunotherapies. I am confident that Immatics’ innovative T cell therapies hold the potential to alter the future therapeutic landscape of solid and hematologic malignancies.”
Additional information about the clinical studies is available at www.immatics.com/clinical-programs/ and www.clinicaltrials.gov.
About Immatics’ Adoptive Cell Therapies
Adoptive Cell Therapy (ACT) is a therapeutic approach that uses natural or engineered T cells to fight cancer. Immatics has developed three innovative, proprietary approaches to produce Adoptive Cell Therapies: ACTengine®, off-the-shelf ACTallo® and the multi-target pilot trial ACTolog®.
About ACTengine®
Immatics’ clinical product class ACTengine® is a personalized approach for patients with advanced solid cancers. Patient’s own T cells are genetically modified to express a novel proprietary TCR cognate to one of Immatics’ cancer targets identified by its proprietary XPRESIDENT® target discovery platform.
About the ACTengine® clinical trials (IMA201, IMA202 and IMA203)
- The primary objective of these clinical studies is to evaluate the safety and tolerability of the ACTengine® approach in target-positive solid cancer patients.
- The secondary objectives are the evaluation of the persistence of T cells in vivo and the assessment of anti-tumor activity.
- Patients are potentially eligible for ACTengine® cell therapy if the target of interest is present on the patient’s tumor as demonstrated by biomarker profiling (IMADetect™).
- Each TCR used in these trials has been selected from the human T cell repertoire and developed using Immatics’ XCEPTOR™ platform targeting highest specificity.
- The ACTengine® T cell products are manufactured at The Evelyn H. Griffin Stem Cell Therapeutics Research Laboratory in collaboration with The University of Texas Health Science Center at Houston (UTHealth).
- Immatics has developed a proprietary manufacturing process, optimized to generate T cell products within a short manufacturing period. TCR-transduced T cells are activated and multiplied outside of the body before being infused into the patient.
Forward-Looking Statements:
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or Immatics’ future financial or operating performance. For example, statements concerning the timing of product candidates and Immatics’ focus on partnerships to advance its strategy are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, uncertainties and factors set forth in filings with the Securities and Exchange Commission (SEC). Nothing in this presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Immatics undertakes no duty to update these forward-looking statements.
Immatics combines the discovery of true targets for cancer immunotherapies with the development of the right T cell receptors with the goal of enabling a robust and specific T cell response against these targets. This deep know-how is the foundation for our pipeline of Adoptive Cell Therapies and TCR Bispecifics as well as our partnerships with global leaders in the pharmaceutical industry. We are committed to delivering the power of T cells and to unlocking new avenues for patients in their fight against cancer.
Immatics’ pipeline consists of two distinct therapeutic modalities of Adoptive Cell Therapies and TCR Bispecifics. Adoptive Cell Therapy programs are developed in collaboration through Immatics US with The University of Texas MD Anderson Cancer Center and co-funded by the Cancer Prevention and Research Institute of Texas (CPRIT). The ACT T cell products are manufactured at the Evelyn H. Griffin Stem Cell Therapeutics Research Laboratory in collaboration with UTHealth.
For regular updates about Immatics, visit www.immatics.com. You can also follow us on Twitter and LinkedIn.
immatics biotechnologies GmbH
Paul-Ehrlich-Str. 15
72076 Tübingen
Telefon: +49 (7071) 5397-0
Telefax: +49 (7071) 5397-900
http://www.immatics.com
Corporate Communications
Telefon: +1 (346) 204-5409
E-Mail: Pamela.Castillo@immatics.com
![]()





