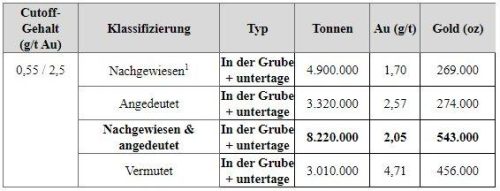
Lonza Announces Construction of a New Small Molecule Manufacturing Complex in Visp
- Lonza will invest CHF 200 million to construct a new manufacturing complex
- The 2000 m2 complex will include a customer dedicated manufacturing line for antibody-drug conjugate payload molecules
- The investment is supported by a long-term collaboration and capital contribution with a major biopharmaceutical partner
- The new manufacturing complex is designed to accommodate future small molecule expansions
Quote from Maurits Janssen, Strategic Business Development Small Molecules, Lonza:
“Supply is critical for our global partner in the oncology field. With this investment, we are enabling the treatment of many cancer patients. Oncology continues to be the leading indication in biopharma and a key driver for highly potent ingredients such as antibody-drug conjugates. In addition, small molecule oncology therapies require specific technologies. These challenges were specifically taken into account when designing this manufacturing complex.”
Quote from Gordon Bates, President and Head of Small Molecules, Lonza:
“This collaboration is a great example of how we are supporting our customers with flexible business models. Our customers developing highly potent and increasingly complex medicines trust us to handle these toxic substances throughout their clinical and commercial lifecycle. The investment to realize this new manufacturing complex for small molecules creates a substantial footprint for accelerating future growth capacity across the range of our small molecule service offering.”
Lonza, a CDMO partner to the biopharma industry, today announced the construction of a new small molecule manufacturing complex at its Visp (CH) site. The new manufacturing complex will occupy an overall footprint of approximately 2000 m2 with six levels of manufacturing space. The complex will offer several opportunities for future expansions supporting the small molecules technologies offering, which include drug substance, particle engineering technologies such as spray drying dispersion, and drug product.
This construction project is supported by a capital contribution and a tailored long-term collaboration with a major biopharmaceutical partner to ensure continuity of supply and flexibility, supporting future demand for their product. The first buildout represents a dedicated manufacturing line for antibody-drug conjugate payload molecules, which is expected to start its first operations in Q3 2023. Upon completion, the manufacturing complex will employ approximately 200 people.
About Highly-Potent API at Lonza
Lonza is a globally leading partner in developing and manufacturing HPAPI, with more than 20 years of experience in safely progressing more than 30 products from early-stage work to late-stage clinical or commercialization. The company has the capabilities in place to safely handle HPAPIs to exposure levels down to 100ng/m3 across all manufacturing scales. HPAPI development and manufacturing is complemented by contained particle engineering and specialized drug products inclusive of oral solid and parenteral options within its global site network.
Click here for more information on Lonza’s HPAPI services and capacity.
About Antibody-Drug Conjugates at Lonza
ADCs are an increasingly important class of HPAPI designed for the targeted treatment of cancer patients. These complex molecules consisting of an antibody, e.g., monoclonal antibody, which has proven highly effective at cell targeting, linked to a biologically active cytotoxic payload for killing cancer cells. Lonza supports an extensive pipeline of ADC development programs and currently produces the majority of commercially available ADC drug products. The company continues to invest in integrated capabilities for developing and producing all components of this increasingly important cancer treatment: cytotoxic payloads, antibodies and the required linkers on one site.
Click here for more information on Lonza’s Antibody Drug Conjugate services and capacity.
Lonza is the preferred global partner to the pharmaceutical, biotech and nutrition markets. We work to prevent illness and enable a healthier world by supporting our customers to deliver new and innovative medicines that help treat a wide range of diseases. We achieve this by combining technological insight with world-class manufacturing, scientific expertise and process excellence. These enable our customers to commercialize their discoveries and innovations in the healthcare sector.
Founded in 1897 in the Swiss Alps, today Lonza operates across five continents. With approximately 14,000 full-time employees, we are built from high-performing teams and of individual talent who make a meaningful difference to our own business, as well as to the communities in which we operate. The company generated sales of CHF 4.5 billion in 2020 with a CORE EBITDA of CHF 1.4 billion. Find out more at www.lonza.com.
Lonza Group Ltd.
Münchensteinerstraße 38
CH4002 Basel
Telefon: +41 (61) 3168638
Telefax: +41 (61) 3169638
http://www.lonza.com
Head of Corporate Communications
Telefon: +41 (79) 599-6260
E-Mail: victoria.morgan@lonza.com
Telefon: +41 (61) 316-8540
Fax: +41 (61) 316-9540
E-Mail: dirk.oehlers@lonza.com
![]()