Kuros Biosciences reports continued increase in MagnetOs U.S. sales
- MagnetOs U.S. YTD sales increase 119% in 2021 compared to 2020
- Kuros expanding MagnetOs range to increase perioperative solutions for surgeons
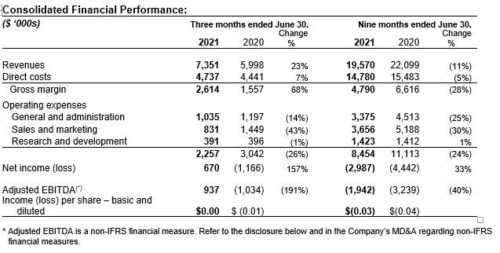
Kuros Biosciences (“Kuros” or the “Company”), a leader in next generation bone graft technologies, today provided an update on its commercial activities. Direct sales of MagnetOs in the U.S. continued to accelerate in Q3 2021, with a year-to-date increase of 119% over the same period in 2020.
Kuros recently showcased new products in the MagnetOs range, including a new 2.5cc unit that is ideal for filling gaps in the spine during posterior cervical fusion procedures, a new formulation of MagnetOs that includes a collagen carrier, and MagnetOs Easypack Putty, a soft and moldable formulation ideal for packing into voids of the skeletal system, which has received 510(k) clearance from the U.S. Food and Drug and Administration.
The addition of new pack sizes, new formulations and new products to the MagnetOs range allows Kuros to offer a more complete portfolio of perioperative solutions to surgeons wanting the therapeutic benefits of MagnetOs bone graft.
The company has also made progress with MagnetOs in Europe this year, signing third-party stocking distributor agreements in nine new territories, including Greece which was signed this week. These agreements add to existing distributor arrangements in the UK and the Netherlands. Clinical cases have already successfully taken place in Spain, Italy, France, Switzerland, Austria, and Denmark. In the UK, the company was recently awarded a major tender to supply biologics under the ‘All Wales Orthopaedic, Trauma and Joint Replacement Framework’, through its UK stocking distributor, Axis Spine Limited. This adds to the existing framework in Scotland which was announced in June. In addition, MagnetOs was also successfully commercially launched in Australia in Q2.
Kuros believes it is well placed to grow its revenues and expects product sales for the full year 2021 to be well above CHF 8.0 million. Cash inflow from collaborations for the full year are expected to be up to CHF 12.0 million.
About MagnetOs
MagnetOs isn’t like other bone grafts. It grows bone even in soft tissue thanks to its unique NeedleGrip surface technology which provides traction for our body’s vitally important ‘pro-healing‘ immune cells (M2 macrophages). This in turn, unlocks previously untapped potential to stimulate stem cells – and form new bone throughout the graft. The growing body of science behind NeedleGrip is called osteoimmunology. But for surgeons and their patients it means one thing: a more efficient and predictable fusion.
US indications statement
MagnetOs is an implant intended to fill bony voids or gaps of the skeletal system, i.e., posterolateral spine. MagnetOs must be used with autograft as a bone graft extender in the posterolateral spine. These osseous defects may be surgically created or the result of traumatic injury to the bone and are not intrinsic to the stability of the bony structure.
EU indications statement
MagnetOs is intended for use as bone void filler for voids and gaps that are not intrinsic to the stability of the bony structure. MagnetOs is indicated for use in the treatment of surgically created osseous defects or osseous defects resulting from traumatic injury to the bone. MagnetOs is intended to be packed into bony voids or gaps of the skeletal system (i.e., extremities, spine, cranial, mandible, maxilla and pelvis) and may be combined with autogenous bone. MagnetOs should not be used to treat large defects that in the surgeon’s opinion would fail to heal spontaneously. In load bearing situations, MagnetOs is to be used in conjunction with internal or external fixation devices.
Forward Looking Statements
This media release contains certain forward-looking statements that involve risks and uncertainties that could cause actual results to be materially different from historical results or from any future results expressed or implied by such forward-looking statements. You are urged to consider statements that include the words “will” or “expect” or the negative of those words or other similar words to be uncertain and forward-looking. Factors that may cause actual results to differ materially from any future results expressed or implied by any forward-looking statements include scientific, business, economic and financial factors, Against the background of these uncertainties, readers should not rely on forward-looking statements. The Company assumes no responsibility for updating forward-looking statements or adapting them to future events or developments.
Kuros Biosciences is a fast-growing leader in the development of spinal fusion biologics that ease the burden of back pain. With locations in the United States, Switzerland and the Netherlands, the company is listed on the SIX Swiss Exchange. The company’s first commercial product, MagnetOs, is a unique synthetic bone graft that has already been used successfully across three continents and in some 4,000 spinal fusion surgeries. The next candidate in the Kuros pipeline is Fibrin-PTH – the first drug-biologic combination for interbody spinal fusions, currently undergoing a Phase 2 clinical trial in the US. For more information on the company, its products and pipeline, visit kurosbio.com.
Kuros Biosciences AG
Wagistrasse 25
CH8952 Schlieren
Telefon: +41 (44) 733-4747
Telefax: +41 (44) 733-4740
http://kuros.ch
Chief Financial Officer
Telefon: +41 (44) 73347-47
E-Mail: michael.grau@kurosbio.com
![]()