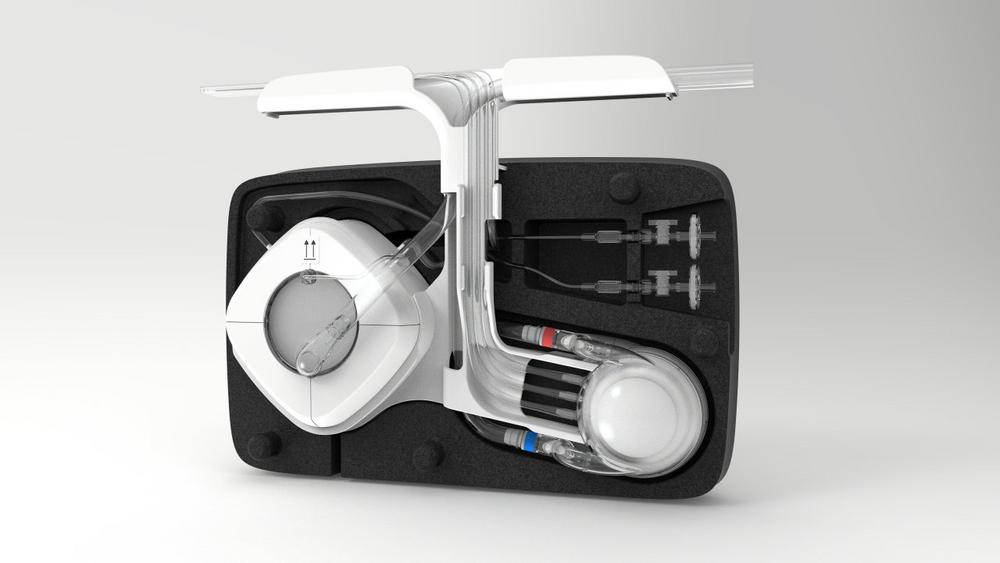
Hemovent’s MOBYO® Gas Exchanger Receives MDR Certification and 28-Day Approval
“I am repeatedly impressed at everything our team manages to achieve in such a short time. The 28-day authorization of the MOBYO® gas exchanger in particular will provide safer patient treatment for our users,” said Dr. Jürgen O. Böhm, CEO & CMO of Hemovent GmbH.
The MOBYO® gas exchanger is part of the MOBYBOX® ECLS system and is also naturally available separately. The innovative design of the MOBYO® ensures extremely low pressure reduction while providing highly efficient gas exchange; the creatively designed cylindrical membrane stacks prevent stagnation areas. This permits gentle blood flow behavior, resulting in greater safety and reliability in patient treatment. The MDR certification for the MOBYO® and the 28-day authorization are further significant steps for the company as it internationalizes its ECLS products.
Hemovent is an emerging medical device company based in Aachen, Germany that has won multiple awards. Its MOBYBOX® product is an extremely portable machine that easily fits in a backpack and is even easier to operate. With this highly innovative ECLS system, Hemovent wants to expand the application area of this life-saving technology for heart-lung support to a fully new dimension. Since 2021, Hemovent has been focusing on growth and aims to shape the future of the portable ECLS system market in intensive care medicine over the long term as a member of MicroPort®, a global medical devices company operating in multiple international markets.
Hemovent GmbH
Pascalstr. 59
52076 Aachen
Telefon: +49 (241) 9901330
http://hemovent.com
Head of Marketing
Telefon: +49 (241) 990133-27
E-Mail: sbastian@hemovent.com
![]()