BDC Laboratories Introduces Software to Simplify Complex GOA Determination for Prosthetic Heart Valves
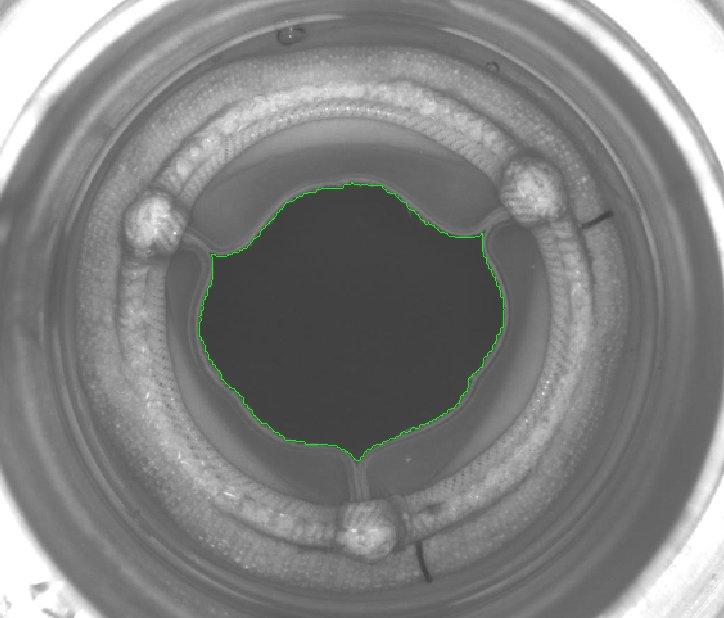
GOA analysis is a multi-dimensional imaging challenge because heart valve substitutes have complex geometries and perform nuanced movements. Statys® GOA is the first purpose-built software for quantitative comparison of heart valve leaflet opening area between hydrodynamic testing and accelerated wear testing. It’s designed to help engineering teams better comply with ISO 5840.
Learn more about the software here: https://www.bdclabs.com/testing-equipment/statys-goa-software/
Statys® GOA automatically analyzes video and still images, overlays boundaries, and determines the GOA for each frame, making analysis efficient, accurate, and simple. Image contrast is crucial in GOA analysis because, without clear differentiation between the leaflet edges and the background, it’s challenging to achieve accurate measurements using edge detection. With multiple algorithms for tailoring analysis and correcting image quality, it’s easy to fix less-than-ideal lighting conditions that traditionally confound these measurements.
With an intuitive interface and automated report generation, it’s easy for anyone on the team to keep track of test results and avoid knowledge transfer gaps.
A Proven Path to GOA Analysis
“This software takes an esoteric, complex measurement and simplifies it,” said Craig Weinberg, PhD, Chief Technology Officer, BDC Laboratories. “It’s a very purpose-developed software. Statys® GOA does one thing really well—and that’s quantifying the opening area of a valve throughout the cardiac cycle.”
Today, some engineering teams create their own GOA analysis algorithms in general-purpose software platforms. With Statys® GOA, BDC Laboratories is leveraging decades of experience to ensure efficient and insightful GOA analysis, even for teams with limited resources.
About BDC Laboratories: Biomedical Device Consultants and Laboratories (BDC Laboratories) offers testing services and products that aid in the mechanical and functional evaluation of Class 2 and Class 3 medical device technologies for regulatory submission. With deep expertise, BDC Laboratories can test, evaluate, and support novel and well-established technologies. Devices include heart valves, heart valve repair technologies, and related stents and grafts. BDC Laboratories is ISO/IEC 17025:2017 accredited and in compliance with Good Laboratory Practices (GLP).
Tentamus Group GmbH
An der Industriebahn 26
13088 Berlin
Telefon: +49 (30) 206038-230
Telefax: +49 (30) 206038-190
http://www.tentamus.com
BDC Laboratories
Telefon: +1 303.456.4665 Ext. 1031
E-Mail: bcarlson@bdclabs.com
Social Media & Content Manager
Telefon: +49 (30) 206038403
E-Mail: sara.pinto@tentamus.com
![]()