-
Immatics weitet klinisches Studienprogramm für adoptive Zelltherapien auf Europa aus
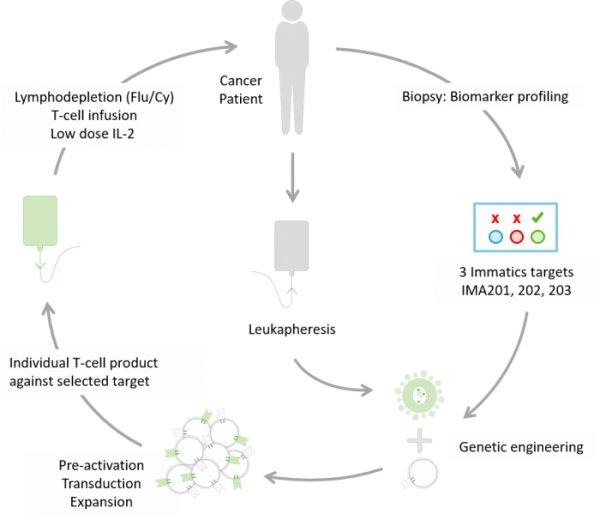
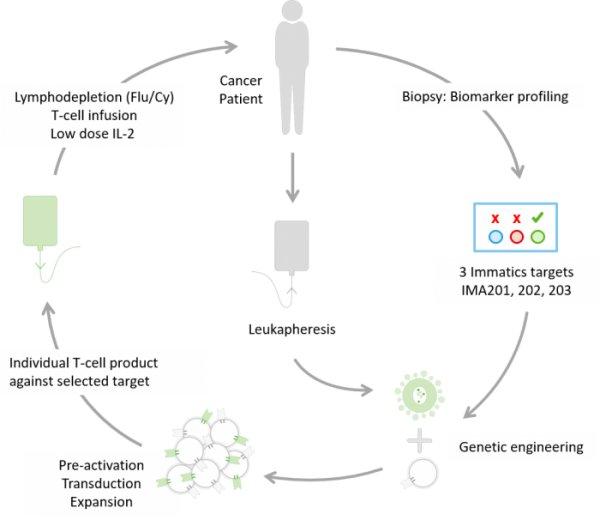
Der erste Patient in Deutschland wurde im Rahmen der Immatics‘ ACTengine® IMA202-101 Studie behandelt Die deutsche Zulassungsbehörde, das Paul-Ehrlich-Institut (PEI), hat den Antrag (CTA, Clinical Trial Application) für eine weitere klinische ACTengine®-Studie in Deutschland genehmigt. Die Studie wird Immatics‘ Produktkandidaten IMA203 untersuchen Drei klinische Studienzentren haben in Deutschland mit der Patienten-Rekrutierung für Immatics‘ ACTengine®-IMA200-Studienprogramme begonnen Immatics N.V. (NASDAQ: IMTX, „Immatics“), ein Unternehmen, das sich auf die Entwicklung und Herstellung von T-Zell-Immuntherapien für die Behandlung von Krebs fokussiert, gab heute die Behandlung des ersten Patienten im Rahmen der IMA202-101 Studie in Europa bekannt. Zuvor hatte das Paul-Ehrlich-Institut (PEI), die Zulassungsbehörde für Zell- und Gentherapien in Deutschland, seine Genehmigung für die Clinical…
-
Immatics Announces European Clinical Expansion of its Adoptive Cell Therapy Programs
First patient has been treated in Germany in Immatics’ ACTengine® IMA202-101 trial German regulatory agency, Paul-Ehrlich-Institute (PEI), granted approval to commence another clinical ACTengine® trial in Germany investigating Immatics’ IMA203 product candidate Three clinical trial sites in Germany have started recruiting patients for Immatics’ ACTengine® IMA200 trial series Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell redirecting cancer immunotherapies, announced today the treatment of the first patient in the IMA202-101 trial in Europe following the Clinical Trial Application (CTA, the equivalent of an IND approval by FDA) approval by Paul-Ehrlich-Institute (PEI), the regulatory body for cell and gene therapies in…
-
Immatics Extends Cell Therapy Manufacturing Collaboration with UTHealth
. Extension of collaboration between UTHealth and Immatics until end of 2024 providing Immatics exclusive access to state-of-the art cGMP manufacturing infrastructure at The Evelyn H. Griffin Stem Cell Therapeutics Research Laboratory Collaboration ensures continued clinical batch supply for all of Immatics’ ongoing and next Adoptive Cell Therapy (ACT) clinical trials in US and Europe Immatics N.V. (NASDAQ: IMTX; “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell redirecting cancer immunotherapies, today announced the extension of its cell therapy manufacturing collaboration with The University of Texas Health Science Center at Houston (UTHealth), in Houston, Texas. The continued collaboration grants Immatics access to UTHealth’s state-of-the-art cGMP…
-
Immatics Announces Board of Directors Additions Following Public Listing on NASDAQ
Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell redirecting cancer immunotherapies, today announced changes to its Board of Directors in conjunction with its recent listing on NASDAQ. Michael Atieh, Paul Carter, Heather Mason and Adam Stone will join the Company’s board as new members. Christof Hettich, L.L.D. will remain a Board Member and Peter Chambré will continue to serve as the Chairman of the Board. Collectively, this group of Directors brings a range of experience and expertise which will be invaluable to Immatics’ continued progress as a leader in T cell receptor (TCR)-based therapeutics. Peter Chambré, Chairman of Immatics’ Board of…
-
Arya Sciences Acquisition Corp. Shareholders Approve Business Combination Transaction with Immatics
Arya Sciences Acquisition Corp. (NASDAQ: ARYA or “Arya”), a special purpose acquisition company sponsored by Perceptive Advisors, and Immatics Biotechnologies GmbH (“Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell redirecting cancer immunotherapies, today announced that its respective shareholders approved the business combination between Arya and Immatics announced on March 17, 2020. None of Arya’s shareholders redeemed their shares in connection with the ARYA shareholder approval. The respective boards of directors of both Arya and Immatics had previously approved the business combination. Following the Arya shareholder vote, Immatics and Arya commenced final procedures towards the closing of the business combination and expect the closing to…
-
Immatics Appoints Cedrik Britten as Chief Medical Officer
Immatics Biotechnologies GmbH, a clinical-stage biopharmaceutical company active in the discovery and development of T cell redirecting cancer immunotherapies, today announced that Cedrik Britten, MD, has been appointed as Chief Medical Officer (CMO) effective June 1, 2020. Trained as a physician, Cedrik Britten will join Immatics with more than a decade of experience in clinical development including his most recent position as Vice President and Head of the Oncology Cell Therapy Research Unit at GlaxoSmithKline. He will be responsible for the management and global development of Immatics’ clinical pipeline. In parallel, Carsten Reinhardt, MD, PhD, the current Chief Medical Officer at Immatics, will assume the newly created role as Chief…