-
NOMAD BIOSCIENCE Extends and Broadens Its Agreements with Research and Commercial Growers, Spain, to Conduct Pilot Field and Greenhouse Trials Aimed at Industrial Manufacturing of Its Protein Products
NOMAD BIOSCIENCE GmbH, Munich, Germany (“NOMAD”) is pleased to announce that it has renewed and extended its development and production agreements with CENTRO TECNOLOGICO NACIONAL AGROALIMENTARIO EXTREMADURA (“CTAEX”), Badajoz, TABACOEX cooperative, Rosalejo, and OLIVOS DE BADAJOZ, Badajoz in Extremadura region of Spain. NOMAD is conducting these activities on behalf of NAMBAWAN Biotech GmbH, Munich, Germany, its spin-off company developing products for non-medical markets. In 2021, CTAEX and TABACOEX conducted first open field and greenhouse trials with NOMAD plant hosts expressing Thaumatin II, a natural high-intensity non-caloric sweetener of plant origin. In 2022, OLIVOS DE BADAJOZ joined the list of collaborators. This year, in addition to Thaumatin II, pilot production of…
-
Nomad Bioscience and Fraunhofer Institute of Cell Therapy and Immunology Extend their Research and Development Agreement
Nomad Bioscience GmbH, Munich, Germany (“NOMAD”) is pleased to announce that the Company has extended its research and development agreement (“Agreement”) with the Fraunhofer Institute of Cell Therapy and Immunology, Leipzig/Halle, Germany (“FRAUNHOFER”) that covers multiple areas of research and early development of NOMAD’s R&D pipeline product candidates, including but not limited to, antibacterial proteins for control of multi-drug and pan-drug resistant pathogenic Gram-negative bacteria as well as antiviral proteins lectins for prevention and early therapy of enveloped viruses including respiratory viruses. The collaboration also includes food additives (sweet and taste modifying proteins) which NOMAD develops on behalf of Nambawan Biotech GmbH, NOMAD’s food spin off company. Agreement provides NOMAD…
-
NOMAD Bioscience receives patents in Japan and Australia for its antibacterials Salmocins
NOMAD Bioscience GmbH has announced that the patent offices of Japan and Australia have issued the decisions to grant patents for NOMAD’s patent applications ‘Bacteriocins for the control of Salmonella enterica, JP2019-551665 and AU2018239310.The patents cover NOMAD’s Salmocins, non-antibiotic antibacterial proteins and the company’s lead product candidates for food and feed safety and veterinary markets. The first patent of this family has already been granted in USA (US11161886B2). Salmonella enterica, Gram-negative bacteria contaminating food products are a major cause of bacterial enteric infections worldwide and the leading cause of deaths due to foodborne bacterial infections. NOMAD product candidates described and claimed in the patent application are antibacterial proteins Salmocins produced in…
-
NOMADS UAB, Lithuania Published a Milestone Research Paper Describing its Chimeric Bacteriocins for Control of Multidrug-Resistant Pseudomonas
NOMADS UAB, Lithuania, NOMAD Bioscience’s wholly owned subsidiary, announces publication in the Nature group journal Scientific Reports (Š. Paškevičius, V. Dapkutė, A. Misiūnas, M. Balzaris, P. Thommes, A. Sattar, Y. Gleba and A. Ražanskienė, “Chimeric bacteriocin S5-PmnH engineered by domain swapping efficiently controls Pseudomonas aeruginosa infection in murine keratitis and lung models” Sci Rep. 2022 April 19; 12:5865. doi: 10.1038/s41598-022-09865-8) of a collaborative research paper describing successful engineering of broadly active chimeric bacteriocins by using domain swapping. The best chimeric molecule, Nomad’s lead product candidate, has broader antimicrobial spectra against Pseudomonas aeruginosa than the natural bacteriocins, and it provides an efficient control of infection in validated murine keratitis and lung infection models.…
-
NOMAD BIOSCIENCE Broadens Its Agreements with Research and Commercial Growers, Spain, to Conduct Pilot Field and Greenhouse Trials Aimed at Industrial Manufacturing of Its Protein Products
NOMAD BIOSCIENCE GmbH, Munich, Germany (“NOMAD”) is pleased to announce that it has renewed and extended its development and production agreements with CENTRO TECNOLOGICO NACIONAL AGROALIMENTARIO EXTREMADURA (“CTAEX”), Badajoz, Spain, TABACOEX cooperative, Rosalejo, and OLIVOS DE BADAJOZ cooperative, Badajoz in Extremadura region of Spain. In 2021, CTAEX and TABACOEX conducted first open field and greenhouse trials with NOMAD plant hosts expressing Thaumatin II, a natural high-intensity non-caloric sweetener of plant origin. This year, OLIVOS DE BADAJOZ joined the list of the collaborators. In addition to Thaumatin II, pilot production of other product candidates, such as bacteriocin ColU, will be conducted. The plant biomass will then be transported to a processing…
-
NOMAD Bioscience Receives GRAS Regulatory Recognition by FEMA for Its Plant-Made Natural Thaumatin II as a Flavour Modifier/Enhancer
NOMAD Bioscience GmbH received a formal GRAS recognition letter from the Flavor and Extract Manufacturers Association (FEMA) in response to NOMAD’s GRAS application describing use of plant-expressed Thaumatin II as a flavour modifier/enhancer (FEMA GRAS 5010). FEMA’s expert panel has affirmed Thaumatin II as a GRAS substance that can be safely used in a wide variety of foods and beverages at an average maximum use level of 7 ppm. Thaumatin II is a natural flavour modifier and non-caloric high intensity sweetener, and NOMAD’s lead product candidate with new superior attributes. GRAS is a facilitated US regulatory marketing allowance pathway for food substances and ingredients that are ‘Generally Recognized As Safe’…
-
Prof. Dr. Yuri (Jurijus) Gleba, CEO and majority shareholder of NOMAD Bioscience has supported Ukrainian Army by donating 100 thousand Euro to reinforce the defense against Russian aggression
Prof. Dr. Yuri (Jurijus) Gleba, renown scientist and entrepreneur, CEO and major shareholder of Nomad Bioscience GmbH, a German biotechnology company, has donated 100 thousand Euro to the Armed Forces of Ukraine to support the defense against Russian aggression. Prof. Gleba also pledged his future support and expressed his gratitude to all researchers and businessmen worldwide who condemned the war of terrorist Russian state against Ukraine and are supporting Ukrainians in their patriotic fight. Dr. A. Gyrych, CSO of NOMAD, Prof. Y. Gleba, and other Nomad colleagues are actively supporting Ukraine by donations, helping refugees, and providing help to their colleagues and other people in Ukraine. Über die Nomad Bioscience…
-
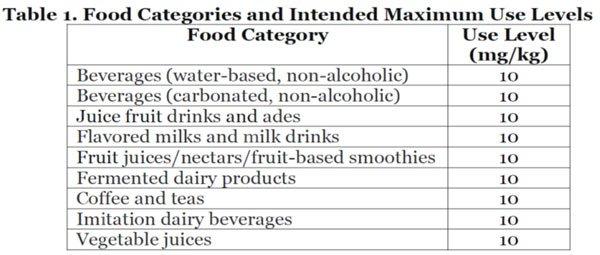
NOMAD Bioscience Extends Its GRAS Regulatory Clearance In USA For Plant-Made Natural Thaumatin II As Sweetener, Amendment GRN 910S
NOMAD Bioscience received a formal ‘no questions’ letter from the US Food and Drug Administration (FDA) in response to NOMAD’s GRAS notice amendment GRN 910S describing broader use of plant-produced Thaumatin II as sweetener. Thaumatin II is natural noncaloric high intensity sweetener under development at NOMAD. GRAS is a facilitated US regulatory marketing allowance pathway for food additives and ingredients that are ‘Generally Recognized As Safe’ under conditions of intended use. The FDA’s response represents the ninth regulatory concurrence from the Agency in response to NOMAD’s GRAS submissions. NOMAD submitted an amendment to the supplement on June 9, 2021, providing additional information regarding the cumulative dietary exposure to the ingredient,…
-
Nomad Bioscience and Fraunhofer Institute of Cell Therapy and Immunology Extend and Broaden their Research and Development Agreement
Nomad Bioscience GmbH, Munich, Germany (“NOMAD”) is pleased to announce that the Company has extended and broadened its research and development agreement (“Agreement”) with the Fraunhofer Institute of Cell Therapy and Immunology, Leipzig/Halle, Germany (“FRAUNHOFER”) that covers multiple areas of research and early development of NOMAD’s R&D pipeline product candidates, including but not limited to, antibacterial proteins for control of multi-drug and pan-drug resistant pathogenic Gram-negative bacteria (Klebsiella, Escherichia, Pseudomonas, Salmonella) as well as antiviral proteins lectins for prevention and early therapy of enveloped viruses including coronaviruses, influenza viruses and immunodeficiency viruses. Agreement provides NOMAD with broader access to FRAUNHOFER expertise and research capabilities in areas of new drug development…
-
NOMAD Bioscience Receives Notice of Allowance from USPTO for Its Patent Application ‚Bacteriocins for control of Salmonella enterica‘
NOMAD has announced that the United States’ Patent and Trademark Office (USPTO) has issued the "Notice of Allowance" for the NOMAD’s patent application “Bacteriocins for control of Salmonella enterica”, U.S. Appl. No. 16/577,484. All filed claims of the application necessary for a broad protection have been allowed. This is the second issued U.S. patent that protects NOMAD’s bacteriocins, non-antibiotic antibacterial biologics and the company’s lead product candidates for medicine, veterinary as well as food/feed safety markets. In 2018, USPTO has awarded NOMAD a broad patent for bacteriocins Colicins, antibacterial biologics for control of Escherichia coli. Salmonella enterica contaminating food products is the main cause of deaths due to bacterial enteric…